Houston, TX, September 30, 2015 — The Texas Heart Institute (THI) announced today that it has successfully implanted three Thoratec® HeartMate 3® Left Ventricular Assist Devices (LVADs) at CHI St. Luke's Health –Baylor St. Luke's Medical Center (Baylor St. Luke's). THI's rich history with LVAD research and the success of the HeartMate 3 implants at Baylor St. Luke's highlight the two organization's dedication to combating heart failure and shaping the future of research and treatment in the field.
"LVADs have become the gold standard for the treatment of end-stage heart failure as destination therapy, or as a bridge-to-transplant," says Steve Singh, MD, Assistant Professor of Surgery at Baylor College of Medicine, and Principal Investigator for the HeartMate 3 clinical trial at Baylor St. Luke's. "Texas Heart Institute and Baylor St Luke's have been at the forefront of innovating and driving the improvements in the technology to make these devices smaller, safer, and more effective for patients suffering from severe heart failure."
The HeartMate 3 LVAD is an implantable mechanical device that helps circulate blood throughout the body. Ventricular assist devices (VADs) are designed to supplement the pumping function of the heart for patients whose hearts are too weak to pump blood adequately on their own. THI was among the first select centers chosen to evaluate the HeartMate 3 LVAD in the MOMENTUM 3 clinical trial. The trial is expected to enroll more than 1,000 patients nationwide.
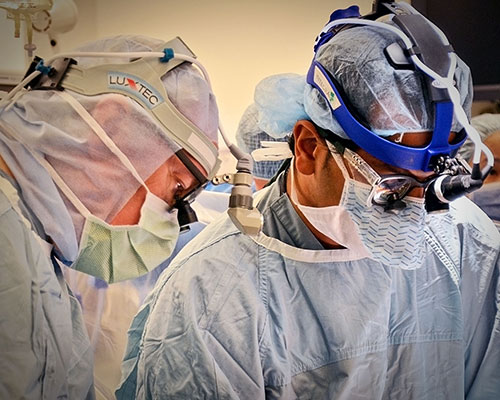
O H "Bud" Frazier, MD and Steve K. Singh, MD implant a HeartMate III. |
"The HeartMate 3 is a paradigm shift in LVAD design, using modulated, full magnetic levitation of the propeller to improve blood flow and hemodynamics," says Dr. Singh. "This hopefully will improve the long-term durability and safety for patients who are living with and benefiting from these devices."
The second HeartMate 3 was successfully implanted in Wesley Guillory Jr. in July of this year. Mr. Guillory was first diagnosed with congestive heart failure in 2008. In February 2015, he required insertion of a pacemaker, after which he elected to enroll in the MOMENTUM 3 trial and was selected to receive the HeartMate 3 device. Prior to implantation of the pump, Guillory would feel shortness of breath upon even minimal exertion, such as carrying his three-month-old daughter. Now, only two months postoperatively, he feels as good as he did prior to his initial 2008 diagnosis.
"The HeartMate 3 has been a significant life changer for me," says Guillory. "I feel great and now I don't have to hold back when I'm interacting with my kids. It's given me my life back."
O.H. Frazier, MD, Chief of the Center for Cardiac Support and Director of Cardiovascular Research at THI, leads the surgical team that implanted the three HeartMate 3s thus far and has been involved in the research behind the LVAD technology almost since its inception.
In 1986, Dr. Frazier performed the first implantation of the HeartMate I LVAD; this device was the first LVAD to be approved by the FDA. His seminal work in the field of LVADs continued with experimental studies that resulted in the first intravascular, implantable continuous flow pump, the Hemopump, which he first implanted in a human in April 1988. Following more than a decade of research, in 2000, he performed the first human implantation of the Jarvik 2000 LVAD (also a continuous flow pump). In 2003, he implanted the first successful HeartMate II device. The HeartMate II pump arose from Dr. Frazier's research and now is the most widely used LVAD in the world. Frazier has performed over 1,300 heart transplants, more than any other surgeon in the world, and implanted more than 900 LVADs. As a result of Dr. Frazier and his team's work, Baylor St. Luke Medical Center continues to be one of the top transplantation and mechanical circulatory support programs in the world.
"It is very gratifying to see the use of the HeartMate 3, particularly since the research to develop this technology began in our laboratory," says Dr. Frazier. "We are hopeful that devices of this type will bring even further advances to the rapidly developing field of blood pump support for the heart failure patient."
* US: Caution: Investigational Device: Limited by Federal United States law to investigational use.