FDA Approves for Permanent Treatment of Advanced Heart Failure
Assist Device Pioneered by
Texas Heart Institute at St. Luke's Episcopal Hospital
Houston, Texas (January 21, 2010)
– The federal Food and Drug Administration today approved a continuous-flow heart-assist device pioneered at the Texas Heart Institute (THI) at St. Luke's Episcopal Hospital (SLEH) for use as a permanent treatment for advanced heart failure.
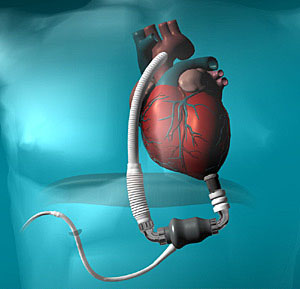 The approval of the pump device, the HeartMate II, follows several years of clinical trials and is seen as a major milestone for patients in the United States. In any given year there are some 250,000 people who suffer from advanced heart failure, while only about 2,000 heart transplants are performed annually in the U.S.
The approval of the pump device, the HeartMate II, follows several years of clinical trials and is seen as a major milestone for patients in the United States. In any given year there are some 250,000 people who suffer from advanced heart failure, while only about 2,000 heart transplants are performed annually in the U.S.
In addition to the need far outpacing the supply of donor hearts, many patients, due to a variety of circumstances such as age and medical complications, are simply not candidates for heart transplants.
The device was previously approved as a way to "bridge" patients until a donor heart could be found. Today's approval of the device as a destination (meaning permanent) therapy means those patients awaiting transplants, or those ineligible for transplants, have a badly needed new treatment option.
Because the HeartMate II is relatively small, requires less invasive surgery, is highly portable and has fewer moving parts than other pumps, it is expected to significantly increase the quality of life for patients. In addition, because of the HeartMate II's small size, it has greatly expanded the population of patients who can be helped by such devices. Studies also have shown that continuous-flow pumps have significant advantages over pulsing pumps, including that they last far longer and don't require patients to undergo replacement procedures as often.
 "This is a huge accomplishment, ushering in a new era of hope for thousands of people," said Dr. O.H. Frazier, THI's Chief of the Center for Cardiac Support and widely considered the leading pioneer in development of heart-assist pumps and the latest continuous-flow technology. "We performed the first implant of the newly designed HeartMate II device in the United States as a bridge to transplant in November 2003 in a young man dying of heart failure. To date we have successfully implanted 150 of the devices – more than any center in the world – both as a bridge to transplant and as participants in the trial to study the device as a permanent solution."
"This is a huge accomplishment, ushering in a new era of hope for thousands of people," said Dr. O.H. Frazier, THI's Chief of the Center for Cardiac Support and widely considered the leading pioneer in development of heart-assist pumps and the latest continuous-flow technology. "We performed the first implant of the newly designed HeartMate II device in the United States as a bridge to transplant in November 2003 in a young man dying of heart failure. To date we have successfully implanted 150 of the devices – more than any center in the world – both as a bridge to transplant and as participants in the trial to study the device as a permanent solution."
The patient who received the first implant subsequently received a heart transplant. Dr. Reynolds Delgado, his cardiologist and Medical Director of Mechanical Support Devices in Heart Failure at THI, said "The concept of an artificial heart has been dreamed about since the 1960s, and finally a practical solution is available that can be used permanently in these desperate patients who have no other options. Most exciting is that some of these patients, after having the device for a few months, actually have healing of their natural heart and recovery of its function, so the device can be removed. This was something never thought possible prior to these devices."
About Heart Failure
The American Heart Association estimates that about 5 million Americans are affected by congestive heart failure, with 550,000 new cases diagnosed each year. The prognosis for patients with advanced heart failure is poor, with projected one-year mortality rates exceeding those of other terminal diseases such as AIDS, leukemia, and lung cancer.1,2 According to the American Heart Association, cardiovascular disease remains the No. 1 cause of death in the United States. Though transplants offer hope for approximately 2,000 advanced heart failure patients each year, over 250,000 patients have had no viable treatment option and are considered at high risk for repeated hospitalizations, severely diminished quality of life and limited life expectancy.3,4 Recent advancements in mechanical circulatory support (MCS) therapy, through the implantation of a ventricular assist device, offer new hope for this advanced heart failure patient population, where there is a critical care need.
HeartMate II as a Treatment Option
The Thoratec® HeartMate® II is the latest FDA-approved left ventricular assist device (LVAD) for destination therapy (DT). A mechanical circulatory support (MCS) device designed to provide long-term cardiac support, HeartMate II is much smaller than previously approved LVADs and is intended for a broad range of advanced-stage heart failure patients, including women. The device can pump up to 10 liters of blood per minute, covering the full output of a healthy heart. It is implanted alongside a patient's native heart and takes over the pumping ability of the weakened heart's left ventricle. HeartMate II is easier to implant than prior devices, and with only one moving part, the HeartMate II is designed to provide exceptional reliability and improved patient quality of life. The device is designed to have a much longer functional life than the previous generation of devices and to operate more simply and quietly.
----------------------
1 Data on file, Thoratec Corporation.
2 Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim, DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, GuptaL, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001 Nov 15;345(20):1435-43.
3 Organ Procurement and Transplantation Network. January 2007
4 Health Research International. U.S. Opportunities in Heart Failure Technologies. March 2004
For more information about THI's research in this area, see Heart Assist Devices and for stories about the use of the HeartMate II, see our multimedia room.
For media inquiries please contact:
Texas Heart Institute
Frank Michel ♦ 713-218-2210
Email: fmichel@heart.thi.tmc.edu
For THI and St. Luke's media profiles, see Public Affairs.



