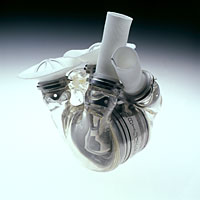
 The AbioCor™ implantable replacement heart is the first completely self-contained total artificial heart. It is the product of 30 years of research, development, and testing conducted by ABIOMED, Inc. and its collaborators, with the support of the National Heart, Lung, and Blood Institute. The AbioCor is designed to sustain the body's circulatory system and to extend the lives of patients who would otherwise die of heart failure. Its unique design allows it to be totally implanted within the body. Unlike the artificial hearts of the past, patients are not tethered to a large, air-pumping console nor do they have wires or tubes piercing their skin.
The AbioCor™ implantable replacement heart is the first completely self-contained total artificial heart. It is the product of 30 years of research, development, and testing conducted by ABIOMED, Inc. and its collaborators, with the support of the National Heart, Lung, and Blood Institute. The AbioCor is designed to sustain the body's circulatory system and to extend the lives of patients who would otherwise die of heart failure. Its unique design allows it to be totally implanted within the body. Unlike the artificial hearts of the past, patients are not tethered to a large, air-pumping console nor do they have wires or tubes piercing their skin.
The AbioCor is intended for use in end-stage heart failure patients whose hearts have irreversible left and right ventricular failure and for whom surgery or medical therapy is inadequate. Currently, heart transplantation is the only proven method of cardiac replacement for extending the lives of such patients; however, there remains a consistent shortage of available donor hearts for transplantation. The Food and Drug Administration has given approval for the initial implantations of the AbioCor, after which it will review the results to determine if the study should be expanded to include more patients, including patients at other medical centers. In the meantime, the FDA and ABIOMED officials have determined that the initial patients to receive the AbioCor must meet the following criteria:
- Have end-stage heart failure.
- Have a life-expectancy of less than 30 days.
- Are not eligible for a natural heart transplant.
- Have no other viable treatment options.
On July 2, 2001, surgeons at Jewish Hospital in Louisville, Kentucky, performed the first implant of the AbioCor in a human patient—a 59-year-old named Robert Tools. Since that time, additional implants have been performed at other hospitals throughout the country, including the Texas Heart Institute.
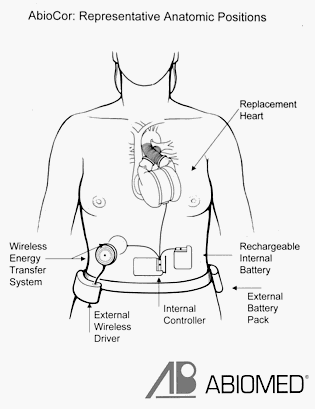 Internal Components
Internal Components
- A thoracic unit (the pump), weighing about 2 pounds. The thoracic unit consists of the artificial ventricles, which contain their corresponding valves, and a motor-driven hydraulic pumping system. The hydraulic pumping system uses pressure to shuttle blood from side to side—in this case, from the artificial right ventricle to the lungs or from the artificial left ventricle to the rest of the body. To create this pressure, the pump's motor rotates at 4000 to 8000 revolutions per minute.
- An internal rechargeable battery, which is an emergency battery that is continually charged by the external power source. The internal battery can provide up to 20 minutes of operation while disconnected from the main battery pack.
- An electronics package, which is implanted in the patient's abdominal area. It monitors and controls the pumping speed of the artificial heart.
External Components
The AbioCor is normally powered by an external console or battery packs. The internal battery will power the pump only when the external power supply is disconnected. Power to the AbioCor is achieved with an energy-transfer device called a transcutaneous energy transmission (TET) system. The TET system consists of internal and external coils that are used to transmit power across the skin. Because tubes or wires do not pierce the skin, the chances of developing an infection are decreased. External battery packs can power the AbioCor for 4 hours.
For more information, you can read an article entitled, "Current Status of the AbioCor Implantable Replacement Heart," which appears in Volume 71 (2001) of the journal Annals of Thoracic Surgery (pp. S147-S149).