|
images courtesy Thoratec Corporation, Inc.
|
The Thoratec HeartMate® II LVAS was developed and tested by Thoratec Corporation, Inc., and the Texas Heart Institute (THI). The HeartMate II has been approved both for use as a bridge to transplantation as well as for destination therapy—as permanent support for advanced heart failure patients who are not eligible for heart transplantation.
January 21, 2010 News Release
FDA Approves Assist Device for Permanent Treatment of Advanced Heart Failure
The federal Food and Drug Administration approved a continuous-flow heart-assist device pioneered at THI for use as a permanent treatment for advanced heart failure.
April 24, 2008 News Release
HeartMate II Approved as Advanced-Stage Heart Failure Treatment Option
THI plays a significant role in clinical studies leading to FDA approval of left-ventricular assist device as a bridge-to-transplantation therapy.
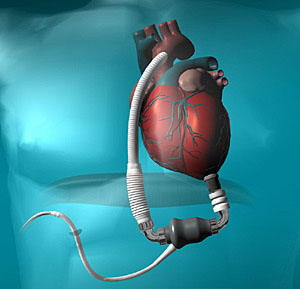
The Pump
The HeartMate II is a high-speed, axial flow, rotary blood pump. As an axial flow device, the HeartMate II produces no pulsatile action. Weighing 12 ounces (about 375 grams) and measuring about 1.5 inches (4 cm) in diameter and 2.5 inches (6 cm) long, it is significantly smaller than other currently approved devices. As such, it may be suitable for a wider range of patients, including small adults and children.
The internal pump surfaces are a smooth, polished titanium. Within the pump is a rotor that contains a magnet. The rotor assembly is rotated by the electromotive force generated by the motor. The rotor propels the blood from the inflow cannula out to the natural circulation. The pump speed can vary from 6,000 rpm to 15,000 rpm, providing blood flow of up to 10 liters per minute.
The pump can run in two operating modes: fixed speed and auto-speed. In fixed-speed mode, the device operates at a constant speed, which can be adjusted via the system monitor. In the auto-speed mode, the pump speed varies in response to different levels of patient or cardiac activity.
External Equipment
External equipment includes a system driver, a power base unit, and a 20-foot power cable, as well as batteries and other accessories. The system driver continuously monitors and controls the implanted motor and shows information regarding alarm conditions.
The power base unit serves as a battery charger and an interface between the system monitor and the implanted pump. The 20-foot power cable allows the system to be operated by AC power. Patients can wear a portable battery pack around their waist, which permits the system to be operated tether-free for 3 hours.